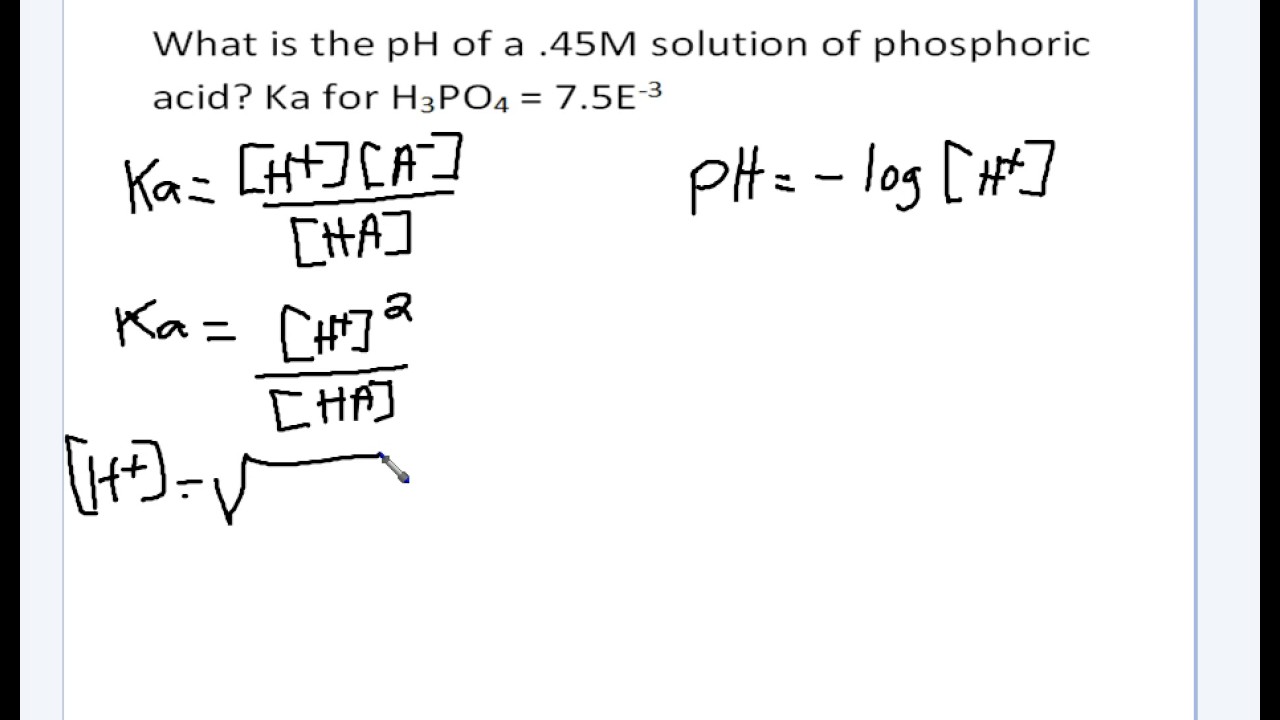
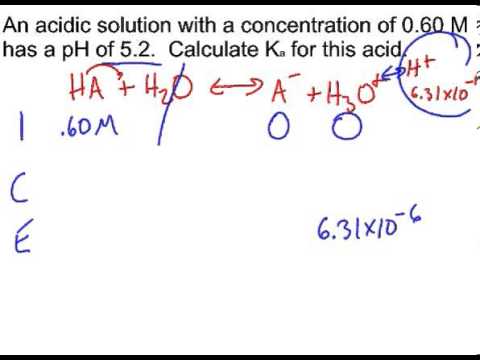
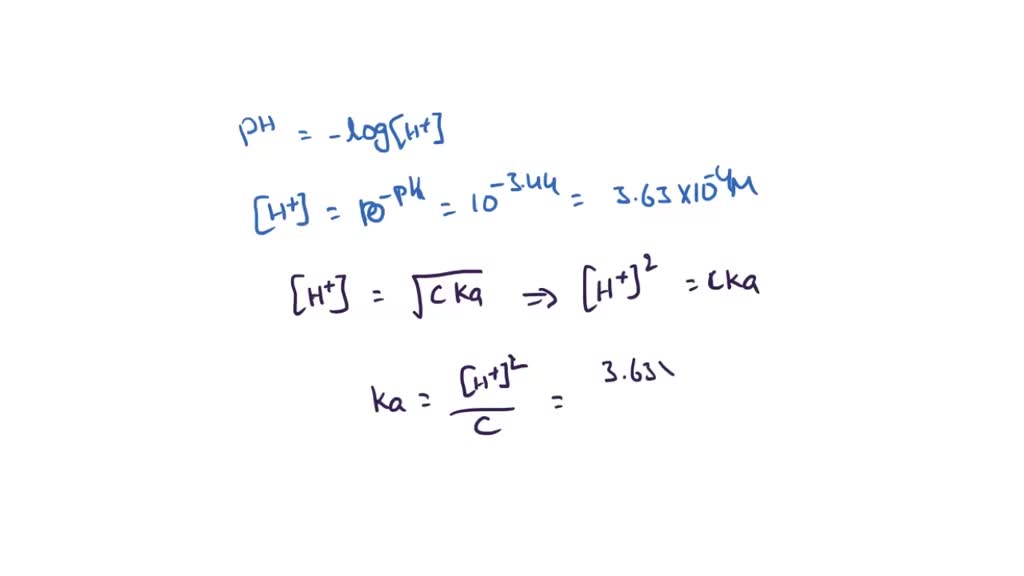Chapter 16C - Calculating Ka from the pH The pH of a 0.10 M solution of formic acid HCOOH at 25 C is 2.38. Calculate Ka for formic acid at | Course Hero

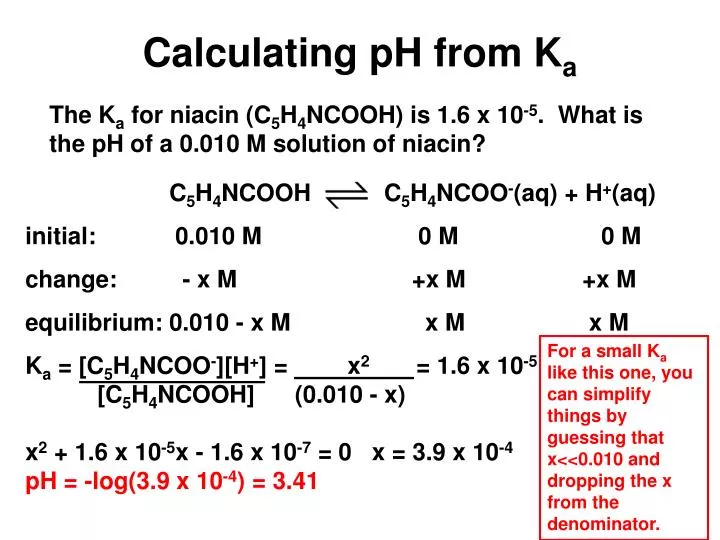
Calculate the `pH` at which an acid indicator with `K_(a) = 1.0 xx 10^(-5)` changes colour when ... - YouTube
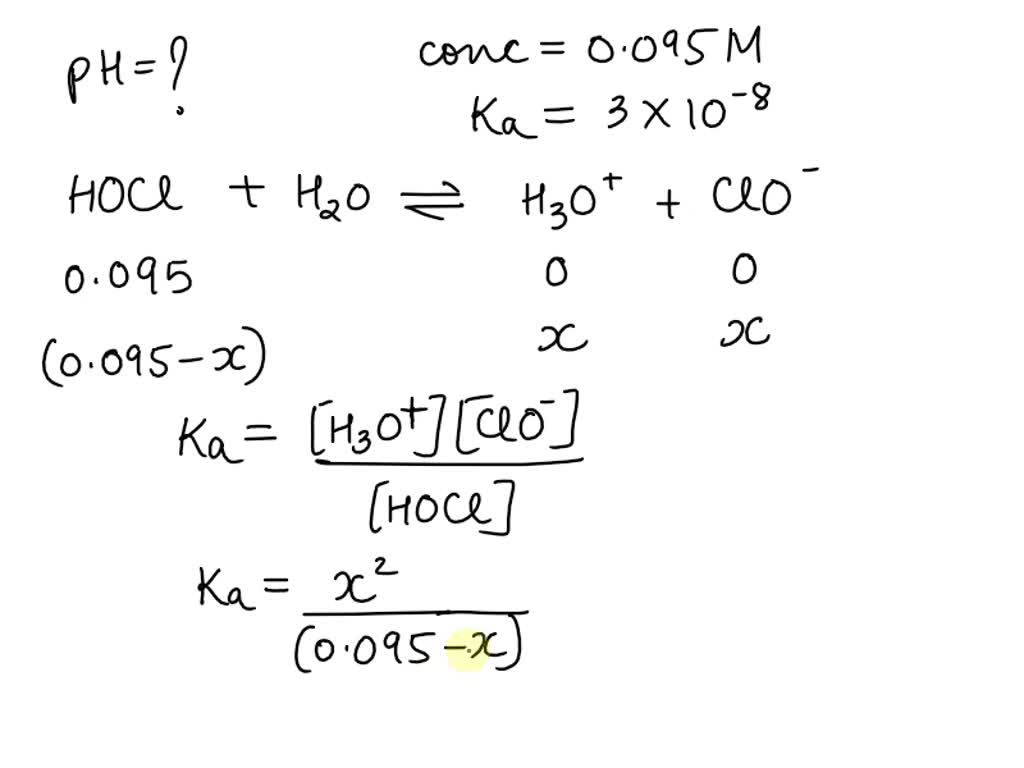
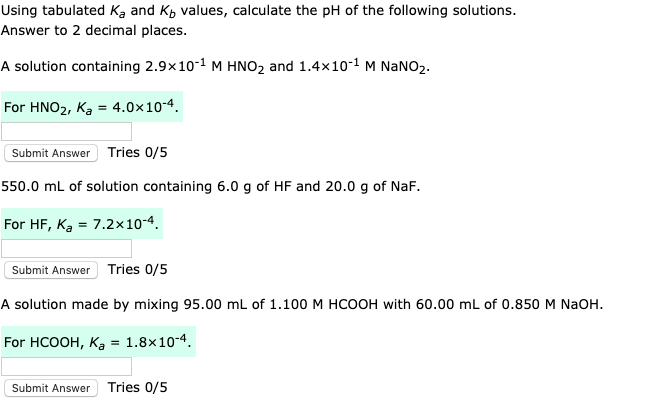
SOLVED: Calculate the pH of 0.095 M hypochlorous acid (HClO), given that Ka = 3.0 x 10-8 Concentration: I think that its 0M, I'm not sure cause above was all the info